Abstract
Background:
Sickle cell disease (SCD) is a chronic blood disorder, which disproportionately impacts Black individuals. Hydroxyurea therapy prevents SCD-related complications; yet it is underutilized, which contributes to further health inequities. Mobile health (mHealth) apps for patients and providers have the potential to improve hydroxyurea adherence. Our objective was to use the RE-AIM framework to evaluate the impact of the COVID-19 pandemic on implementation and effectiveness of patient and provider hydroxyurea (HU) mHealth apps (InCharge Health app and HU Toolbox app, respectively) in two large academic medical centers.
Methods:
Patient-level data (n=46) were collected between 11/2019-7/2020. Adherence was measured by calculating Percentage of Days Covered (PDC) from prescription records over 24 weeks before implementing the mHealth apps and during 12 and 24 weeks of implementation. As a response to the COVID-19 pandemic and to reduce virus spread, both sites temporarily suspended non-emergent clinical activities. During implementation, Site A clinics shut down for approximately 2 months (3/2020-5/2020) and Site B clinics for 7 months (3/2020-10/2020). To better understand contextual factors associated with mHealth implementation, we purposively sampled participants according to app use level (high vs low) and conducted semi-structured interviews with adult SCD patients, providers (physicians, nurse practitioners, and physician assistants), administrators, and research staff between 6/2020 and 3/2021.
Results:
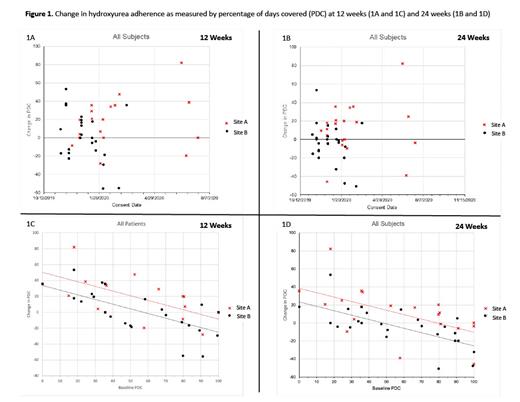
A total of 46 patients participated and contributed to the PDC hydroxyurea adherence data. Mean change in PDC, adjusted for baseline PDC, was greater at Site A than at Site B (12-weeks: difference = 16.59%, p=.01, Figure 1A; 24-weeks: difference = 15.08%, p=.01, Figure 1B).
Eleven patients (mean age 26.2 years old, 64% males, 100% Black, 73% HbSS, 45% low app users) and 11 providers (mean age 36.7 years old, 73% females, 36% Black, 54.5% physicians, average 8 years in practice, 36% low app users) completed the semi-structured interviews across the 2 sites. Site B was more affected during the COVID-19 pandemic where patients had difficulty obtaining hydroxyurea and other challenges related to reaching their providers and clinic setting for non-urgent or emergent reasons. In addition, participants with lower baseline hydroxyurea adherence level, as measured by PDC, had a more remarkable improvement in their PDC values at 12 weeks (Figure 1C) and 24 weeks (Figure 1D) Additional qualitative data focused on the implementation process were collected from 3 administrators, and 4 research staff. Among patients, both high and low app users reported the pandemic was a barrier to getting needed care (e.g., difficulty getting to hospital/clinic) and obtaining hydroxyurea; this was particularly a concern among low app users at Site B. Among providers, all but 2 high app users reported the pandemic did not impact app use, but nearly all low users perceived the pandemic to be a barrier to using the provider app because fewer patients came to clinic for maintenance SCD visits during the COVID-19 pandemic. Low users also reported the pandemic would negatively impact continued use of the app. Administrators and research staff also said reduced in-person clinic visits were a barrier to app implementation.
Conclusions:
mHealth apps are promising tools for improving hydroxyurea adherence among adolescents and adults with SCD. Contextual data show that some patients who experienced challenges accessing healthcare during COVID-19 pandemic have also experienced challenges navigating mHealth implementation. A focus on removing barriers to mobile apps use during care disruptions will likely improve patient and provider mHealth apps implementation, and ultimately, reduce health inequities for this vulnerable population. Our findings suggest that strategies such as including an mHealth facilitator has the potential to help addressing some of these implementation challenges.
Note: Sherif Badawy and Lisa DiMartino are co-first authors with equal contribution
Badawy: Sanofi Genzyme: Consultancy; Bluebird Bio Inc: Consultancy; Vertex Pharmaceuticals Inc: Consultancy. Shah: Alexion: Speakers Bureau; Bluebird Bio: Consultancy; CSL Behring: Consultancy; Emmaus: Consultancy; GBT: Consultancy, Research Funding, Speakers Bureau; GLG: Consultancy; Guidepoint Global: Consultancy; Novartis: Research Funding, Speakers Bureau. Hankins: Bluebird Bio: Consultancy; UpToDate: Consultancy; Vindico Medical Education: Consultancy; Global Blood Therapeutics: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal